Obtain Regulatory Approvals for EU Market Access for IVDD 98/79/EC
Ensure your in vitro diagnostic medical device meets the essential requirements of the In Vitro Diagnostic Directive (IVDD), including quality system, design, manufacturing, packaging, and labeling requirements. We can help you navigate the CE Marking process, prepare your technical file, establish a monitoring system and incident reporting system, document risk, and prepare a Declaration of Conformity for your products within the scope of the Directive:
- Self-testing devices (products deemed for home-use)
- Reagents/reagent products (Annex II, List A Devices; Annex II, List B Devices)
- All other medical devices to be used in-vitro for the examination of blood or tissue specimens
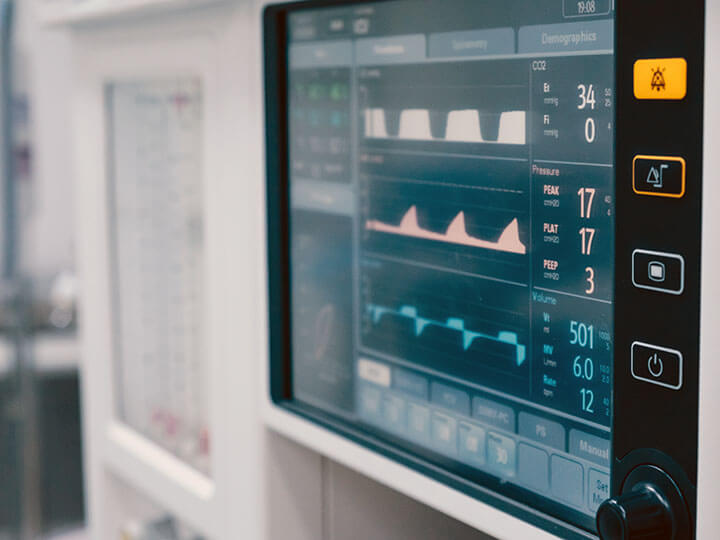
In Vitro Diagnostic Medical Equipment Overview
Knowledge Center
Download the latest information from our medical device compliance experts.
In-Situ and Manufacturer Location EMC Testing Webinar
IEC 81001-5-1 and Cybersecurity for Medical Devices Webinar
Six Compliance-Related Questions About Connected Home Healthcare Devices
Accelerated Stress Testing for Medical Devices
Machine Learning and Artificial Intelligence (AI) in Medical Devices: Webinar | Fact Sheet
Creation of IEC 60601-1 4th Edition
IEC 60601-1-2 Ed. 4.1 Overview of Requirements
Medical OEM Wireless Coexistence Testing
Biocompatibility Risk Assessment and Evaluation Plans
For more expert papers, recordings, and presentations, visit our Medical Resources hub.
Related Links
- ETL Mark The Industry's Fastest Certification Program
- Search and Buy Medical Device Standards
- Reese's Law – ANSI/UL 4200A-2023
- My Test Central
- Directories
- Certification Marks
- Global Research & Certification
- Medical Podcast - Compliance with Clarissa
- Satellite Client Test Program
- Planning for Quality through Disruptions
- Intertek's world-class team of Medical Experts
*The Intertek legal entities that provide medical device management system certification services (including ISO 13485 and MDSAP) and Notified Body services (MDR 2017/745 and MDD 93/42/EEC) do not provide any consulting services. Clients who have used other Intertek legal entities’ consulting services are not eligible to receive management system certification services or Notified Body services from Intertek.

