Understanding Compliance in Modern Patient Monitoring Devices
31 Jul 2025
A New Era for Patient Monitoring Requires a New Approach to Risk and Regulation
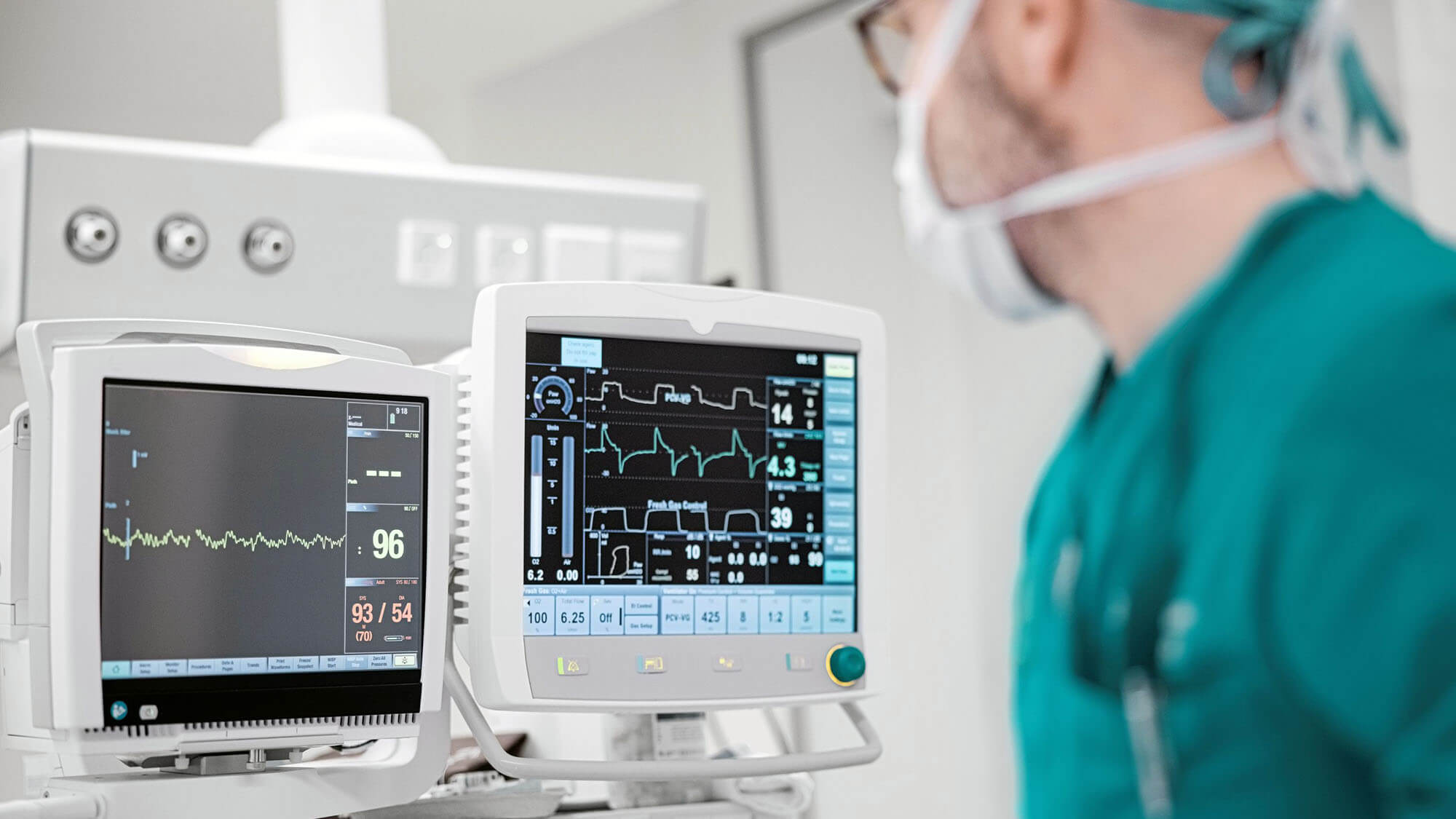
As the healthcare landscape shifts toward remote and real-time care, patient monitoring devices have evolved significantly. No longer limited to large, bedside units in hospital rooms, today’s monitoring technologies are wearable, mobile, and increasingly integrated with consumer platforms like smartphones and tablets. This transformation is exciting, but it also brings a host of new challenges, particularly in compliance, risk management, and regulatory testing.
From Bedside to Wearable: The Changing Face of Patient Monitoring
Traditional patient monitors were relatively simple in structure: tabletop or wall-mounted units that measured and displayed basic vitals like heart rate, blood pressure, or oxygen saturation. These devices typically included a display and a handful of sensors connected directly to the patient. Modern devices, however, are much more compact and often consist of small, body-worn components that transmit data wirelessly to a central display, often a commercial tablet or smartphone.
This shift has created opportunities for more accessible care, but it also demands a more nuanced understanding of compliance. Devices now operate outside traditional hospital environments and often connect to a range of accessories or third-party systems, each with their own implications for testing and certification.
Understanding the Standards Landscape
At the heart of patient monitor compliance lies the IEC 60601 family of standards, which provides the global framework for medical electrical equipment. Most projects begin with IEC 60601-1, the general standard that addresses basic safety and essential performance. However, depending on the device's design and use case, additional collateral and particular standards are often required. Collateral standards such as IEC 60601-1-6 (usability) and IEC 60601-1-8 (alarm systems) apply broadly. Others, like IEC 60601-1-11 and IEC 60601-1-12, address home healthcare and emergency medical environments respectively – scenarios increasingly common with modern patient monitors.
Particular standards further define requirements based on specific functions. For example, non-invasive blood pressure measurements fall under IEC 80601-2-30, while invasive blood pressure measurements must meet IEC 60601-2-34. Similarly, ECG monitoring may fall under IEC 60601-2-25 for diagnostic use, -2-27 for bedside monitoring, or -2-47 for ambulatory monitors. Functions such as respiratory gases monitoring, body temperature monitoring and blood oximetry measurements will fall under ISO 80601-2-55, ISO 80601-2-56, ISO 80601-2-61, respectively. If a device monitors multiple parameters, IEC 60601-2-49 may apply.
It’s essential that manufacturers understand these distinctions early. A device may appear to fall under a single particular standard, but as accessories and intended uses are evaluated, multiple standards often become relevant.
Preparing for the Future: The IEC 80601-2-86 Draft
A significant development in this area is the forthcoming IEC 80601-2-86 standard, currently in draft form. This new standard aims to unify the fragmented ECG standards (-2-25, -2-27, and -2-47) into a single, comprehensive framework. It will also incorporate construction and mechanical requirements from older standards such as AAMI EC12 and EC53, which govern aspects like electrodes and lead wires. By consolidating requirements across diagnostic, monitoring, ambulatory ECGs and the constructions of lead wires, the -2-86 standard is expected to simplify compliance and testing efforts, particularly for manufacturers working across multiple device types or product families.
The Role of Risk Management in Compliance
Compliance isn’t just about passing a checklist of tests; it’s about demonstrating that the device is safe and effective in the context of its intended use. This makes risk management a critical part of both the design and evaluation processes. For patient monitors, risk management becomes even more important due to the complexity of the systems involved. Devices may need to interact with multiple accessories or support various configurations across different environments. Alarm systems must respond to both clinical and technical failures, requiring thorough risk analysis to determine how each condition should be detected and signaled.
Testing labs rely heavily on manufacturers’ risk management files when developing test plans. These documents inform everything from worst-case configuration testing to how alarm conditions are triggered during simulations. A strong, continuously updated risk file ensures that testing aligns with real-world use and mitigates potential safety issues.
Avoiding Delays: When to Engage with Compliance
One of the most common mistakes manufacturers make is delaying compliance planning until too late in the development cycle. This often leads to design revisions, repeated testing, and missed regulatory deadlines. Whether it’s a hardware component that doesn’t meet performance requirements or a software feature that lacks proper documentation, late-stage changes can be costly.
Integrating compliance early, preferably at the design input phase, enables engineering teams to anticipate testing requirements, design for regulatory success, and minimize surprises. It also allows for collaborative engagement with testing labs to ensure alignment on applicable standards and expectations. Even though a full compliance evaluation may not be required at the design stage, partnership with a trusted Assurance, Testing, Inspection, and Certification agency throughout product design life cycle for assurance and preliminary design review activities is a great way for manufacturers to ensure a proper adherence to standards at every stage of the process.
Building Stronger Products Through Collaboration
Successful patient monitoring products are the result of careful engineering, regulatory foresight, and close collaboration between manufacturers and test labs. As the category continues to evolve – spanning clinical, home, and emergency environments – the regulatory landscape will continue to grow in complexity.
By engaging early, applying the right standards, and using risk management as a guiding tool, manufacturers can reduce delays, avoid costly redesigns, and ensure their devices are both innovative and safe. In a field where technology changes quickly, but patient safety remains paramount, compliance is a critical part of the product development journey.

